Advanced Laser-Based technology for the preparation of biological tissues and materials in plastic embedded sections
Methodological Aspects of an Advanced Technology purchased by Patho-Logica (supported by the Israeli Innovation Authority).
Dr. Emmanuel Loeb
Case report:
- Objective: To evaluate histopathological changes in goat blood vessels following Implant + Filler implantation, using a semi-quantitative analysis and morphometry.
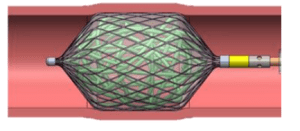
- The device: A vascular embolization device, intended to control hemorrhaging due to aneurysms, vascular tumors and arteriovenous malformations.
- Device scheme:



- Histopathology: A histopathological change in goat blood vessels were evaluated 90-days following device implantation, using a semi-quantitative analysis and morphometry to evaluate percent of vessel recanalization and total percent of occluded area.
- Gross: A vein with an obstruction of 1.5 cm and no apparent other pathological changes (see the right picture).
- Slide Preparation: Plastic sections (6 microns thick) were cut using an advanced Laser microtomy, put on glass slides and stained with Hematoxylin & Eosin (H&E) for histopathology analysis.
- Results: The laser microtomy enabled the evaluation and understanding of the structure and cellular assembling of the obstruction.
- The results had a great impact on the device development process and generated more funding for the project.
- The company is working on a scientific publication of the histopathology results once the device will commercial.
Results:
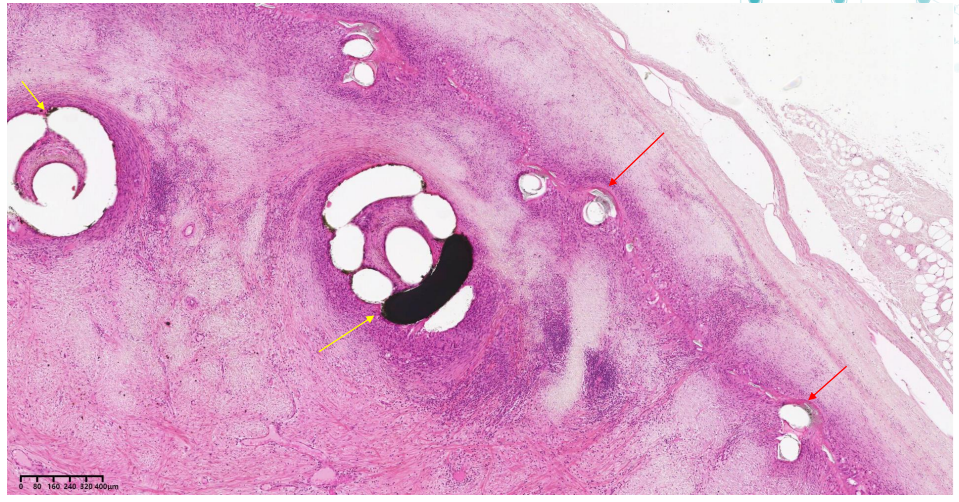
A cross section within the vein device showing the device frame (red arrow), coils (yellow arrow) and tissue ingrowth. X0.9, H&E stain.
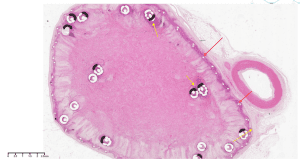

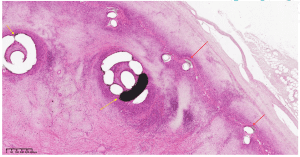
Results:
A cross section within the vein device showing the device frame (red arrow), coils (yellow arrow) and tissue ingrowth. X2, H&E stain.

Summary
- Laser microtomy is a fast and easy cutting method of undecalcified hard tissue and a broad range of implants and biomaterials.
- The laser dissection method enables a 6-14 micron thick section which provide the pathological evaluation a detailed histological feature in plastic embedded material including high power magnifications.
- The cross sections are clean with almost no artifacts.
- In this case of medical device evaluation as represents here the method helps to demonstrate and study the impact of an invasive medical device both in the context of efficacy and safety.