A Hexokinase 2 Modulator for Field-Directed Treatment of Experimental Actinic Keratoses
Vered Behar, Hadas Pahima, Adi Kozminsky-Atias, Nir Arbel, Emmanuel Loeb, Max Herzberg and Oren M. Becker
Overexpression of hexokinase 2, and its binding to VDAC1 on the outer mitochondrial membrane of cancer cells, is key to their metabolic reprogramming to aerobic glycolysis, which enables them to proliferate. We describe Comp-1, an allosteric small molecule that selectively detaches hexokinase 2 from the mitochondria. Detachment of hexokinase 2 reduces glycolysis and triggers apoptosis in cancer cells, without affecting hexokinase 1-expressing normal cells. The anti-cancer activity of Comp-1 was demonstrated in the UVB- damaged skin model in SKH-1 mice. Topical treatment with Comp-1 led to 70% reduction in lesion number and area. This in vivo efficacy was obtained without local skin reactions or other safety findings. Mechanism- related pharmacodynamic markers, including hexokinase 2 and cleaved caspase 3 levels, are affected by Comp-1 treatment in vivo. Good Laboratory Practice toxicology studies in minipigs for 28 days and 13 weeks established no systemic toxicities and minimal dermal reaction for once-daily application of up to 20% and 15% ointment strengths, respectively. Thus, Comp-1 may address a significant unmet medical need for a non-irritating efficacious topical actinic keratosis treatment.
Journal of Investigative Dermatology (2018) 138, 2635e2643; doi:10.1016/j.jid.2018.05.028
INTRODUCTION
Solar UV radiation is the primary cause of skin cancer. Sun- light, especially UVB (range 280e320 nm), acts as a tumor initiator and tumor promoter and is able to damage DNA directly (Madan et al., 2010). Non-melanoma skin cancer, including basal cell carcinoma and cutaneous squamous cell carcinoma (SCC), is the most common form of skin cancer. Actinic keratosis (AK) is on the continuum of transformation from normal skin to SCC and is often referred to as SCC in situ (Ro¨ wert-Huber et al., 2007). If untreated, a small fraction of AKs may progress to SCC involving deeper tissues, metasta- ses, and even death (Oppel and Korting, 2004).
One of the key characteristics of many types of cancer cells, including SCC cells, is their increased rate of glucose uptake and breakdown (glycolysis). Accordingly, to support their growth and proliferation, their metabolism is often reprogrammed from oxidative phosphorylation to aerobic glycolysis, a phenomenon known as the Warburg effect (Hay, 2016; Lunt and Vander Heiden, 2011). The first step in glycolysis is catalyzed by hexokinases (HKs) that phosphor- ylate glucose to glucose-6-phosphate. There are four HK isozymes, the most abundant being HK1 and HK2. HK1 is expressed in most normal adult tissue. In contrast, the expression of HK2 under normal conditions is very limited (Wilson, 2003). Moreover, under normal conditions, the subcellular distribution of HK1 and HK2 is different, with HK1 associated mainly with mitochondria and HK2 present primarily in the cytoplasm (John et al., 2011).
Unlike the absence or low expression of HK2 in the ma- jority of normal tissue, this enzyme is highly expressed in many cancer types, addressing the greater metabolic demand typical of proliferating cancer cells (Patra et al., 2013; Pedersen et al., 2002). High HK2 levels correlate with poor disease prognosis (Peng et al., 2008; Rho et al., 2007; Smith, 2000) and are required for oncogenic transformation despite possible continuous expression of HK1 (Patra et al., 2013).
Both HK1 and HK2 attach to the mitochondria by binding to VDAC1 on the cytosolic side of the outer mitochondrial membrane (Pastorino and Hoek, 2008). The VDAC1 channel allows passage of ions and metabolites, such as adenosine triphosphate (ATP), nicotinamide adenine dinucleotide, and Ca2þ, thus enabling the metabolic cross-talk between the mitochondria and the rest of the cell. VDAC1, too, is over- expressed in many cancer types and plays an important role in cancer progression (Shoshan-Barmatz et al., 2015; Shoshan-Barmatz and Mizrachi, 2012).
While the binding of HK1 to VDAC1 is strong and continuous, the binding of HK2 to VDAC1 is much weaker, and alternates between a cytoplasmic and a mitochondrial-bound states (John et al., 2011). This dynamic process is regulated by the metabolic and energetic requirements of the cells and provides them with significant advantages. First, VDAC1-bound HK2 protects cells from apoptosis, thus enabling their longevity (Gottlob et al., 2001; Majewski et al., 2004; Pastorino et al., 2002). Second, by binding to VDAC1, HK2 gains privileged access to ATP synthesized in the mitochondria, its preferred source for substrate utilization (Pastorino and Hoek, 2008; Wilson, 2003). Finally, this as- sociation results in reduced sensitivity to feedback inhibition by the glucose-6-phosphate product (John et al., 2011; Wilson, 2003). Taken together, the selective dissociation of HK2 from VDAC1 should trigger apoptosis in cancer cells, making it a promising anti-cancer strategy (Krasnov et al., 2013). HK2 has been proposed as a potential target for anti-cancer therapy and a few HK2 inhibitors were recently published (Krasnov et al., 2013; Li et al., 2017a, 2017b; Lin et al., 2016).
Here we present a selective small molecule modulator of HK2’s interaction with the mitochondria (Comp-1, Figure 1), which is a highly potent synthetic derivative of methyl jasmonate, a well-documented plant stress hor- mone with some reported anti-cancer activity (Goldin et al., 2008; Krasnov et al., 2013). Comp-1 does not affect the binding of the normal isoform, HK1, to the mitochondria, thus, representing a potentially well- tolerated anti-cancer drug for AK and cutaneous SCC, as well as for other tumor types.
RESULTS
Comp-1 selectively detaches HK2 from the mitochondria in vitro.
The activity of Comp-1 in a cell-free assay was evaluated using microscale thermophoresis (MST) analysis, which is based on altered movement of a protein in a temperature gradient when bound to other molecules. The HK enzymes were first allowed to bind with VDAC1 to form the VDAC1/ HK complexes. The addition of Comp-1 selectively dissoci- ates HK2 from VDAC1 in a dose-dependent manner with a half maximal inhibitory concentration (IC50) of 0.092 mM. In contrast, Comp-1 does not affect the VDAC1/HK1 complex (Figure 2a).
The cellular effect of Comp-1 was demonstrated in human skin SCC A431 cells (which express both HK1 and HK2). Cells were incubated for 2 hours with increasing concentra- tions of Comp-1, followed by mitochondrial isolation and Western blot (WB) analysis (Figure 2b). Semi-quantification of the WB reveals that there was a dose-dependent reduc- tion of mitochondrial-bound HK2 levels, with an IC50 of approximately 0.8 mM. (Figure 2c). This occurred without changes to total cellular HK2 levels (Supplementary Figure S2 online, Supplementary Material online), suggesting a direct effect on the binding of HK2 to the mitochondria; in contrast, HK1 association with the mitochondria is not affected by Comp-1 in a similar experimental setup (Supplementary Figure S3 online, Supplementary Material). Comp-1 reduces cellular ATP levels by as much as 50% after 2-hour incuba- tion in A431 cells, demonstrating the energetic outcome of HK2 dissociation from the mitochondria (Figure 2d).
Comp-1 treatment results in apoptosis induction. This was demonstrated in two ways: reduction of cytochrome C levels in the mitochondrial fraction within 2 hours of treatment, as evaluated by WB and semi-quantification (Figure 2e); and an increase in cellular levels of cleaved caspase-3 within 6 hours of treatment (Figure 2f). These two early events are in line with a caspase-dependent apoptotic mechanism (Kvansakul and Hinds, 2015).

Figure 1. Chemical structure of Comp-1.
The effect of Comp-1 on cellular viability was demon- strated in 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H- tetrazolium-5-carboxanilide assay in A431 SCC cells and the human AK line HT-297.T, resulting in IC50 values of 0.014 and 0.228 mM, respectively. Further, Comp-1 was evaluated in colony-forming assay in a panel of 57 patient-derived tu-
mors and cancer cell lines, where it exhibited a strong anti- clonogenicity effect (IC50 < 2 mM) in 70% of tested cancer types. Dose-response curves for a subset of these tumor models are shown in Figure 2g. HK2 protein levels in each of these 57 tumor models were determined by WB, semi- quantified, and normalized to a (0e1) scale. Figure 2h shows a statistically significant correlation (P ¼ 0.0002) be- tween cellular HK2 levels and Comp-1 IC50 values. The complete list of tumor models tested in colony-forming assay and 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazo- lium-5-carboxanilide is shown in Supplementary Table S1 (online).
To note, Comp-1 does not affect the catalytic activity of either HK1 or HK2, nor does it affect the kinetic parameters, KM or Vmax, for either substrates, ATP or glucose (Supplementary Figure S4 online, Supplementary Material), further supporting the idea that Comp-1 is an allosteric modulator of the VDAC1/HK2 complex.
Efficacy in UVB-damaged skin model in SKH-1 hairless mouse.
The in vivo efficacy of Comp-1 was tested in a mouse model of chronic UVB exposure. Forty-eight SKH-1 hairless female mice were chronically exposed to UVB radiation for 16 weeks according to the scheme in Figure 3a. By that time, >60% of the animals had developed at least one lesion. Three days after the final irradiation dose, the mice were
randomly assigned to one of four treatment groups (n ¼ 12 mice/group) and a 50-day treatment phase started. Treatment groups included: vehicle, 2.5% or 5% Comp-1 ointment applied once-daily for 50 days, or 40% Comp-1 topical so- lution applied once-daily for the first 5 days of every 3-week cycle (total of three treatment cycles).
The three Comp-1 treatment groups, 2.5%, 5%, and 40%, showed significant efficacy, lowering the number of lesions relative to vehicle by 65%, 70%, and 72%, respectively (P <
0.0001) (Figure 3b). A similar reduction of a 66%e70% was also found in the total area of the lesions relative to vehicle (P < 0.0001) (Figure 3c).
The dose-dependent effect in the animals that received active drug is more pronounced at the time of onset of the effect, which was delayed by approximately 2 weeks in the 2.5% group (Figure 3d). Lesion numbers per individual mouse during the treatment period are presented in Supplementary Figure S5 (online).
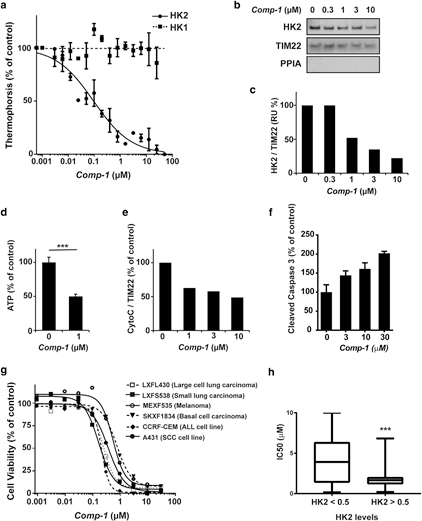

Figure 2. In vitro activity of Comp-1
(a) Dissociation of VDAC1/HK complexes by Comp-1, microscale thermophoresis assay. Mean :::1 standard error of mean, n ¼ 3 independent studies. (b) Cellular detachment of HK2 from the mitochondria in A431 cells following 2-hour treatment, WB of the mitochondrial fraction. PPIA, marker for cytosol. TIM22, mitochondria loading control. (c) Semi- quantification of WB (b). (d) ATP levels following 2-hour treatment with Comp-1 in A431 cells. (e, f) Apoptosis induction in A431 cells. (e) Semi- quantification of cytochrome C in WB of the mitochondrial fraction following 2-hour treatment. (f) Cleaved caspase-3 following 6-hour treatment (ELISA). (g) Colony-forming assay of Comp-1 on patient-derived tumors and cell lines. (h) HK2 levels in 57 cell lines and patient-derived models analyzed by WB and correlated to their IC50 values. ATP, adenosine triphosphate; HK, hexokinase; IC50, half maximal inhibitory concentration; WB, Western blot.
Skin appearance and histopathologic analysis following. Comp-1 treatment.
There were no safety signals or adverse local skin reactions of any kind in any of the Comp-1 treatment groups from day 1 through day 50. Specifically, there were no erythema or edema, no abnormalities in body weight, and no adverse clinical signs in any of the groups, suggesting a promising safety profile for Comp-1. Representative photographs of the treated dorsal skins from each group at the end of the 50-day treatment period are shown in Figure 4a. Histopathologic analysis of the UVB-exposed skins dem- onstrates epidermal thickening (Figure 4b), and the expected
SCC morphology of elongated keratinocyte cords-like struc- tures towards the dermis (Figure 4c), in agreement with pre- vious descriptions of this model (Chaquour et al., 1995; Kligman and Kligman, 1981). Comp-1 treatment results in a thinner epidermis (Figure 4d), which was histologically similar to the skin of na¨ıve, noneUVB-exposed, control mice (Figure 4e).
Comp-1 reduces mitotic index and increases apoptosis in vivo.
The extent to which cells undergo active proliferation was evaluated using a semi-quantitative histopathologic analysis of the epidermis layers on day 50. The analysis demonstrates that Comp-1 significantly reduces the mitotic index in the treated skin (Figure 5a). Furthermore, immunohistochemistry (IHC) analysis of cleaved caspase-3, a marker for early apoptosis, shows a statistically significant induction of apoptosis in Comp-1etreated mice (Figure 5b) to levels similar to those seen in the epidermis of na¨ıve hairless mice.
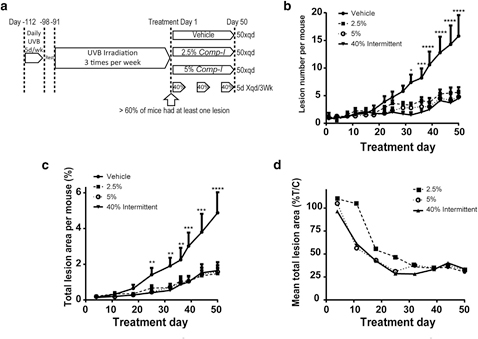

Figure 3. Comp-1 efficacy on UVB- damaged skin in hairless SKH-1 mice.
(a) Study design schematics. n ¼ 12 per group. (b) Number of lesions per mouse per treatment area (6 cm2) over time (mean ב: standard error of mean [SEM]). (c) Total lesion area per mouse per treatment area (6 cm2) over time (mean ב: SEM). (d) Mean total lesion area presented as % of the mean vehicle-control treated group. P values compare the 40% treatment group to the vehicle-control. *P ::; 0.05, **P ::; 0.01, ***P ::; 0.001, ****P ::; 0.0001.
Effect on the pharmacodynamic markers HK1 and HK2
IHC analysis of tissue microarrays from human cutaneous SCC biopsies reveals that HK1 and HK2 levels in the epidermis differ between normal skin and cutaneous SCC. While high HK1 levels and low HK2 levels characterize normal epidermis, the reverse is seen for cutaneous SCC (Figure 6a), in line with published data of high HK2 levels in other cancer types (Patra et al., 2013).
IHC analysis was performed on skin tissues from the in vivo UVB-exposed mice, assessing HK protein levels as potential mechanism-based pharmacodynamic markers. In line with the human data, the epidermis of na¨ıve control mice stained strongly positive for HK1 and negative for HK2 (Figure 6b). In contrast, the epidermis of UVB-exposed mice exhibited the opposite pattern (Figure 6c, vehicle group), that is, strong HK2 and weak HK1 staining, similar to human cutaneous SCC. Fifty-day treatment with 5% Comp-1 re-established a profile that resembles normal skin, in which HK2 staining levels are low (Figure 6c, Comp-1 group).
To assess whether the treatment-induced pharmacody- namic marker switch from HK2 to HK1 occurs early, a small, not statistically powered, satellite study was conducted with similar 16-week UVB-exposed mice. These were treated for only 1 or 2 days with 5% Comp-1 or vehicle. Figure 6d shows that compared to the vehicle-treated mice, which exhibit high HK2 and low HK1 levels due to the UVB irradiation, treatment with Comp-1 for as little as 2 days led to a dramatic decrease in HK2 protein levels in the treated skin, similar to the low HK2 levels present on day 50 of the main study. Treatment for 1 day did not yield this effect (data not shown).
In vivo safety
In addition to Comp-1’s selectivity to HK2 versus HK1 discussed, Comp-1 demonstrates only weak interaction (IC50 > 1 mM) with 98% of the proteins in a panel of 63 off-targets (Supplementary Table S2 online, Supplementary Material). This lack of cross- reactivity is expected to contribute to a favorable safety profile invivo. Comp-1 formulated as a topical ointment completed two repeat-dose Good Laboratory Practice toxicology studies in minipigs, where the drug was applied once-daily to 10% of their body surface area for 28 days at strengths up to 20% w/w and for 13 weeks at strengths up to 15% w/w. No treatment-related systemic changes were detected in either study, and the No Observed Adverse Effect Level for systemic toxicity in both studies was the highest dose tested. In both studies the only notable findings were limited erythema with a mean severity score of “very slight” to “slight/well-defined” that was of similar magnitude and severity in all treatment groups, including the vehicle-treated group. In general, this limited skin reaction appeared within the first 2 weeks of the studies, increased up to week 4 and remained stable or showed a tendency to a slight improvement thereafter. On occasion crust/scabs accompanied the erythema. These findings appeared to be reversible with cessation of the treatment. A maximum tolerated dose for the local application of VDA-1102 was not reached, even at the highest dose tested (20% or 15%, respectively).
DISCUSSION
We present here a targeted approach to treat cancer with an allosteric modulator that selectively detaches HK2 from the mitochondria, without affecting HK1 association, resulting in glycolysis reduction and induction of apoptosis in the ma- lignant cells. Comp-1’s anti-proliferative effect in vitro is correlated with HK2 protein levels in the cells. Specifically, Comp-1 exhibits broad anti-cancer activity in vitro as well as in vivo efficacy in the UVB-damaged skin mouse model. The in vivo efficacy of Comp-1 (70% reduction in lesion count) is similar to the reported efficacy of ingenol mebutate in a similar mouse model (Cozzi et al., 2012). Pharmacodynamic analysis of HK isoform levels in the skin of treated mice confirms Comp-1’s selective mode of action: reducing the high HK2 levels characteristic of the disease state to low HK2 levels similar to those in normal skin. This effect was demonstrated as early as 2 days after initiation of treatment. Unlike most other approved AK drugs that cause significant skin irritation, the efficacy of Comp-1 is delivered without irritation. Good Laboratory Practice toxicology studies in minipigs with a topical ointment formulation of Comp-1 administered once daily for 28 days and 13 weeks estab- lished no systemic toxicities and minimal dermal reaction for
doses of up to 20% and 15%, respectively.
Tolerability for topical AK treatments is a major consid- eration in the management of this disease. Due to field cancerization that is brought about by prolonged exposure to the sun, AK patients require repeat treatments during their lifetime, often over large areas of skin. Lesion-directed treatments, such as cryosurgery and laser-based therapies, are not suitable for treatment of multiple lesions over large areas. Current efficacious field-directed treatments (including 5-fluorouracil, imiquimod, ingenol mebutate, and photodynamic treatment) are frequently associated with severe local skin reactions, which range from mod- erate inflammation to necrosis. These reactions are painful and unsightly, leading to reduced treatment compliance. Comp-1, which demonstrates efficacy, safety, and dermal tolerability in vivo, may address a significant unmet med- ical need for subjects with AK. Comp-1 is in clinical developed as a topical treatment for AK, currently in a phase 2b trial. It is also being developed as a systemic treatment for other malignant diseases.
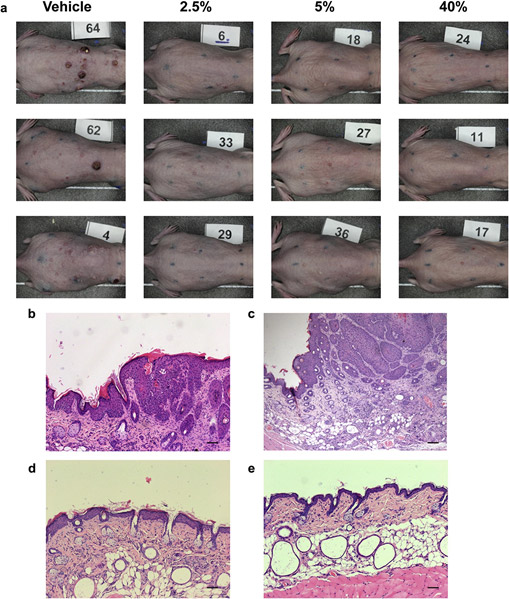

Figure 4. Skin appearance and histopathologic analysis
following Comp-1 treatment. (a) Representative photographs of UVB-damaged mouse skin from all treatment groups on day 50 (three mice per group). The treatment area was marked on the back of the mice by a black eight-point rectangle tattoo. (bee) Representative photographs of hematoxylin and eosin staining of treated dorsal skin sections taken on day 50 showing (b) epidermal thickening and (c) squamous cell carcinoma morphology in UVB-exposed, vehicle-treated mouse; compare to thin epidermis in (d) 5% treated mouse; and (e) na¨ıve mouse. Scale bar ¼ 20 mm.
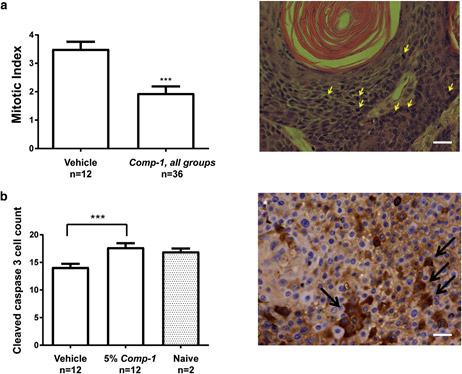

Figure 5. Comp-1 reduces cell proliferation and induces apoptosis in vivo (day 50 data)
Mitotic index quantification of the epidermis, vehicle group (n ¼ 12) versus all Comp-1 treatment groups (n ¼ 36). Quantification included the number of mitotic cells per x40 high-power field, 7 different fields per slide, as evaluated from hematoxylin and eosinestained slides. A sample image is shown. (b) Semi-quantification of cleaved caspase-3epositive cells, performed similarly as in (a). n ¼ number of mice. ***P ::; 0.001. A sample image is shown. Scale bar ¼ 20 mm.
MATERIALS AND METHODS
A431 (human skin SCC) and HT-297.T (human AK) cell lines were purchased from ATCC (Manassas, VA) and were maintained in DMEM supplemented with 10% fetal calf serum, 4 mM L-Gln, 1 mM sodium pyruvate, 100 U/mL penicillin, and 1 mg/mL streptomycin. Human HK1 and HK2 plasmids were purchased from Addgene (Cambridge, MA) and were purified from Escherichia coli. VDAC1 was purified from sheep liver mitochondria as described (Arbel et al., 2012). Human skin tissue microarray (SK208 and SK 805) were purchased from US Biomax Inc (Rockville, MD), which attests that all human materials was obtained under institutional approval of experiments and with written informed patient consent.
Microscale thermophoresis assay
Studies were conducted on Nanotemper Monolith NT.115 (Munich, Germany). HK1 and HK2 were labeled using Monolith NT.115 Protein Labeling Kit BLUE-NHS. As a preliminary study to form the VDAC1-HK complexes, various concentrations of VDAC1 were added to 67 nM HK1 or 71 nM HK2 (for HK-VDAC1 direct binding curves see Supplementary Figure S1 online, Supplementary Material). The preferred VDAC1 concentration was determined to be 1.5 mm. For dissociation studies, HKs were pre-incubated with VDAC1 for 10 minutes and increasing concentrations of Comp-1 were added for an additional 10 minutes. Microscale thermopho- resis default parameters were used and analysis was with Nano- temper analysis software. All studies were done at room temperature in assay buffer of 20 mM Tris, 10 mM glucose, 10 mM MgCl2, and 1% DMSO pH 7.5.
WB
A431 cells were treated for 2 hours at 37oC with various concen- trations of Comp-1 in medium without fetal calf serum and 2 mM L- Gln. Mitochondria were isolated using Mitochondria Isolation Kit (Thermo Fisher Scientific, Rockford, IL) followed by WB.
Semi-quantification of the WB was done with Imagequant TL soft- ware, version 8.1 (GE Healthcare Life Sciences, Little Chalfont, UK). TIM22 was used as a loading control for mitochondria. Cyclophilin A (PPIA) was used as a cytosolic marker.
The following antibodies were used: anti-rabbit HK1 (C-2024S) and HK2 (C-2876S) from CST (Danvers, MA), anti-rabbit PPIA (ab41684; Abcam, Cambridge, MA), anti-rabbit TIM22 (14927-1-AP, Proteintech Group Inc., Rosemont, IL), and anti-rabbit cytochrome C (PA1118, Boster Biological Technology, Pleasanton, CA). Detection was performed using secondary antibodies: peroxidase-conjugated Affinipure goat anti-rabbit IgG (HþL) or goat anti-mouse IgG (HþL) (111-035-003 and 115-035-003; Jackson ImmunoResearch, West Grove, PA), and development was performed using the ECL kit WesternBright ECL (K-12045-D10; Advansta, Menlo Park, CA).
ATP Determination
Cellular ATP levels were determined using CellTiter-Glo Lumines- cent Cell Viability Assay kit (Promega, Madison, WI) following 2 hours of treatment of A431 cells at 37oC.
Apoptosis
Apoptosis induction was determined by semi-quantification of cyto- chrome C levels in the mitochondrial fraction analyzed by WB, following 2 hours of treatment with Comp-1 in A431 cells. Cleaved caspase-3 levels were analyzed following 6 hours of Comp-1 treatment in A431 cells, using PathScan Cleaved Caspase-3 Sandwich ELISA kit (CST).
Cell viability assays
For 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5- carboxanilide assays, cells were plated in 96-well plates (5,000 cells per well) for 24 hours. Cells were then incubated with different concentrations of Comp-1 in triplicate for 72 hours in DMEM sup- plemented with 0.5% fetal calf serum and 5 mM glucose. Cell viability was evaluated using 2,3-bis-(2-methoxy-4-nitro-5- sulfophenyl)-2H-tetrazolium-5-carboxanilideebased colorimetric.
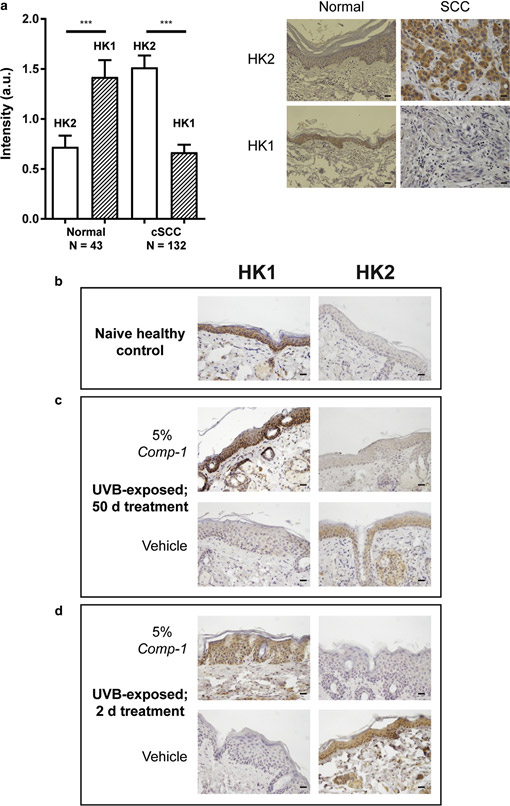

Figure 6. IHC of HK1 and HK2 as pharmacodynamic markers for the action of Comp-1
in skin epidermis. (a). Quantification of HK1 and HK2 staining intensity (arbitrary scale 0e4) in healthy versus cutaneous SCC from human skin microarray samples. n ¼ number of samples. ***P ::; 0.001. Sample images are shown, scale bar ¼ 20 mm. (bed) HK1 or HK2 staining in slides from the UVB-exposed mouse model treated with Comp-1. (b) Skin from na¨ıve mice
(c) UVB-exposed skin samples following 50 days of treatment with 5% Comp-1 or vehicle. (d) Skin following 2 days of treatment showing a pattern similar to that seen in (c). Scale bar ¼ 20 mm. HK, hexokinase; IHC, immunohistochemistry; SCC, squamous cell carcinoma.
assay kit (Biological Industries Ltd., Kibbutz Beit-Haemek, Israel). The optical density was measured by an Infinite 200 ELISA reader at 450 nm and reference was measured at 630 nm (Tecan, Ma¨nnedorf, Switzerland). Colony-forming assay was performed by Charles River Labora- tories (Freiburg, Germany) in 96-well plate format according to a modified two-layer soft agar assay. The origin of the tumor xenografts has been described (Fiebig et al., 1989). The list of tumors used in this study is detailed in Supplementary Table S1.
UVB-damaged skin model in SKH-1 mice
All animal experiments were performed according to guidelines of the
National Institutes of Health and the Association for Assessment and Accreditation of Laboratory Animal Care, following The Israel Board for Animal Experiments approval no. IL-13-08-145. Female SKH-1 mice were obtained from Charles River Laboratories (Wilmington, NC). Mice were treated and managed at Pharmaseed Ltd (Ness Ziona, Israel). The UVB-induced skin damage model was adopted from previous publi- cations (Cozzi et al., 2012; Dinkova-Kostova et al., 2008; Phillips et al., 2013; Rebel et al., 2001) according to the scheme in Figure 3a. Mice were exposed to 1.25 times the minimal erythemal dose of UVB at each exposure. For details see the Supplementary Material.
Topical formulation
The formulation ingredients for vehicle, 2.5%, and 5% Comp-1 ointment for the mouse studies consisted of: 32% Gelucire44/14 (Gattefosse´, Saint-Priest, France) and 68% combined Labrasol (Gattefosse´, Saint-Priest, France) with corresponding amount of Comp-1 (w/w). New ointments were prepared every 2 weeks. The 40% topical solution was prepared just prior to use by mixing (w/w) 60% propylene glycol (Sigma, St Louis, MO) with 40% Comp-1.
IHC, histology, and semi-quantitative analysis of IHC slides Histopathologic analysis and IHC was performed according to standard protocols. The following primary antibodies were used: rabbit anti-HK1 and rabbit anti-HK2 (C35C4 and C64G5; CST) and rabbit anti-cleaved caspase 3 (PP229; Biocare Medical, Concord, CA). The secondary antibody was goat anti-rabbit IgG(HþL) (474- 1506; KPL, Gaithersburg, MD). Semi-quantitative analysis was done in a blinded manner by a single experienced pathologist scoring x40 high-power fields, seven different fields per slide.
In vitro selectivity panel
All experiments were performed by NovaScreen Biosciences Cor- poration, a PerkinElmer company (Hopkinton, MA). Radioligand binding assays measured the ability of Comp-1 to inhibit binding of radiolabeled ligands to their respective receptors and were per- formed for all receptors (including the classes of G-proteinecoupled receptors, ion channels, and transporters). The cyclooxygenase, tyrosine kinase, and phosphodiesterase enzyme assays were per- formed using LabChip technology and run on the EZ Reader II platform. All data presented in Supplementary Table S2 are the mean of duplicate measurements.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism, version
5.03 (Graphpad Software, San Diego, CA).
ORCID
Vered Behar: https://orcid.org/0000-0002-0711-7856
CONFLICT OF INTEREST
All authors, except EL and MH, are employees at Vidac Pharma, Ltd, Israel. EL is an employee at Patho-Logica Ltd, Israel and was not a paid consultant. MH is a stock holder at Vidac Pharma and the inventor of Comp-1.
ACKNOWLEDGMENTS
We wish to thank Varda Shoshan-Barmatz for her continued technical help and advice through the early stages of this work.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www. jidonline.org, and at https://doi.org/10.1016/j.jid.2018.05.028
REFERENCES
Arbel N, Ben-Hail D, Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem 2012;287:23152e61.
Chaquour B, Seite´ S, Coutant K, Fourtanier A, Borel JP, Bellon G. Chronic UVB- and all-trans retinoic-acid-induced qualitative and quantitative changes in hairless mouse skin. J Photochem Photobiol B 1995;28: 125e35.
Cozzi S-J, Ogbourne SM, James C, Rebel HG, de Gruijl FR, Ferguson B, et al. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol 2012;132:1263e71.
Dinkova-Kostova AT, Jenkins SN, Wehage SL, Huso DL, Benedict AL, Stephenson KK, et al. A dicyanotriterpenoid induces cytoprotective en- zymes and reduces multiplicity of skin tumors in UV-irradiated mice. Biochem Biophys Res Commun 2008;367:859e65.
Fiebig HH, Winterhalter B, Berger DP, Lo¨ hr GW. Combined in vitro/in vivo test procedure with human tumor xenografts for anticancer drug develop- ment. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al 1989;165: 522e4.
Goldin N, Arzoine L, Heyfets A, Israelson A, Zaslavsky Z, Bravman T, et al. Methyl jasmonate binds to and detaches mitochondria-bound hexokinase. Oncogene 2008;27:4636e43.
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 2001;15: 1406e18.
Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 2016;16:635e49.
John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS One 2011;6:e17674.
Kligman LH, Kligman AM. Histogenesis and progression in ultraviolet light- induced tumors in hairless mice. J Natl Cancer Inst 1981;67:1289e93.
Krasnov GS, Dmitriev AA, Lakunina VA, Kirpiy AA, Kudryavtseva AV. Tar- geting VDAC-bound hexokinase II: a promising approach for concomitant anti-cancer therapy. Expert Opin Ther Targets 2013;17:1221e33.
Kvansakul M, Hinds MG. The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis 2015;20:136e50.
Li W, Gao F, Ma X, Wang R, Dong X, Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating Hexokinases II-mediated glycolysis. Oncotarget 2017a;8:32586e99.
Li W, Zheng M, Wu S, Gao S, Yang M, Li Z, et al. Benserazide, a dop- adecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase
Lin H, Zeng J, Xie R, Schulz MJ, Tedesco R, Qu J, et al. Discovery of a novel 2, 6-disubstituted glucosamine series of potent and selective hexokinase 2 inhibitors. ACS Med Chem Lett 2016;7:217e22.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic re- quirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27:441e64.
Madan V, Lear JT, Szeimies R-M. Non-melanoma skin cancer. Lancet 2010;375:673e85.
Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 2004;16: 819e30.
Oppel T, Korting HC. Actinic keratosis: the key event in the evolution from photoaged skin to squamous cell carcinoma. Therapy based on pathoge- netic and clinical aspects. Skin Pharmacol Physiol 2004;17:67e76.
Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr 2008;40:171e82.
Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 2002;277:7610e8.
Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexo- kinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013;24: 213e28.
Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Bio- phys Acta 2002;1555:14e20.
Peng S-Y, Lai P-L, Pan H-W, Hsiao L-P, Hsu H-C. Aberrant expression of the glycolytic enzymes aldolase B and type II hexokinase in hepatocellular carcinoma are predictive markers for advanced stage, early recurrence and poor prognosis. Oncol Rep 2008;19:1045e53.
Phillips J, Moore-Medlin T, Sonavane K, Ekshyyan O, McLarty J, Nathan C- AO. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol Head Neck Surg 2013;148:797e803.
Rebel H, Mosnier LO, Berg RJ, Westerman-de Vries A, van Steeg H, van Kranen HJ, et al. Early p53-positive foci as indicators of tumor risk in ultraviolet-exposed hairless mice: kinetics of induction, effects of DNA repair deficiency, and p53 heterozygosity. Cancer Res 2001;61: 977e83.
Rho M, Kim J, Jee CD, Lee YM, Lee HE, Kim MA, et al. Expression of type 2 hexokinase and mitochondria-related genes in gastric carcinoma tissues and cell lines. Anticancer Res 2007;27:251e8.
Ro¨ wert-Huber J, Patel MJ, Forschner T, Ulrich C, Eberle J, Kerl H, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol 2007;156(Suppl. 3):8e12.
Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. The mito- chondrial voltage-dependent anion channel 1 in tumor cells. Biochim Biophys Acta 2015;1848:2547e75.
Shoshan-Barmatz V, Mizrachi D. VDAC1: from structure to cancer therapy. Front Oncol 2012;2:164.
Smith TA. Mammalian hexokinases and their abnormal expression in cancer. Br J Biomed Sci 2000;57:170e8.
Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular local- ization and metabolic function. J Exp Biol 2003;206:2049e57.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit

